To find a solution to how to increase IVF success, we need to know and understand the reasons of IVF failure.
Our patients often have more than one reason why their pregnancies haven’t happened yet or haven’t progressed to the full term. Otherwise, first IVF attempts at their local clinics would have already led to a result, and we would never have met.
To be able to solve or correct each reason of previous IVF failures we stick to the principle “one challenge — one solution”.
When you have a big project, you tend to split it in several smaller projects with smaller goals, which lead to one big goal. The smaller goals here are: eggs, blastocystsBlastocyst — the stage that a human embryo normally reaches on day 5-6 of development. This is the stage on which the embryo implantation process begins., chromosomally normal blastocysts, prepared uterus, ready implantation windowImplantation window is a time frame under 72 hours when special receptors are present on the surface of endometrium, which can recognize receptors on the surface of embryos to start the implantation process., and finally — a chromosomally normal blastocyst for a ready endometriumEndometrium — the lining covering the uterine cavity in the right timing.
One big goal here is a baby.
The reasons for IVF failure and pregnancy loss can be divided into two groups: embryonic and maternal.
Embryonic reasons for IVF failure and pregnancy loss mean that something is some reason inside the embryo. Due to these internal reasons the embryo is not viable and cannot develop, even in the best maternal conditions.
Maternal reasons for IVF failure and pregnancy loss include reasons within the female organism and can be explained by hormonal, immune or uterine factors.
I. Overcoming embryonic reasons for pregnancy loss and IVF failure

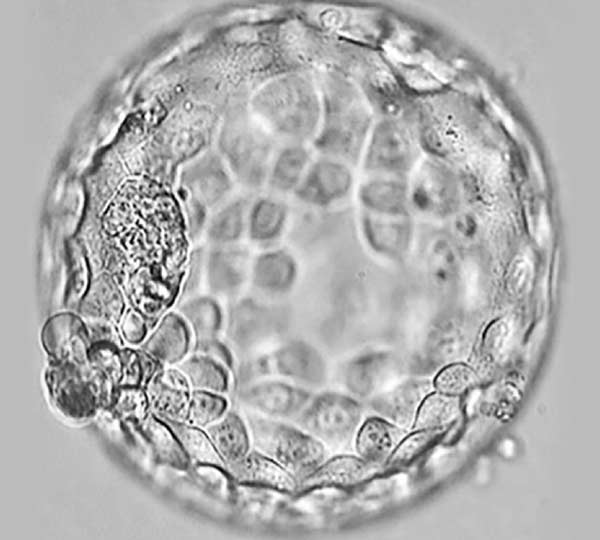
Picture 1. Who is responsible for the outcome of IVF: a woman or an embryo?
Embryonic reasons for pregnancy loss and IVF failure are the most powerful and at least 75% responsible for the outcome of the fertility treatment and pregnancy.
I.1. Genetic situation in human embryos
— Human embryos of parents after the age of 35 often have an abnormal set of chromosomes [Fragouli et al., 2012] and, strange as it may sound, it is normal;
— An abnormal set of chromosomes in the embryo almost always leads to a pregnancy loss at the pre-implantation stage (implantation failure) or at the post-implantation stage (miscarriage) [Scott at al., 2012];
— An abnormal set of chromosomes is almost always of maternal origin, so the embryo gets it from the egg;
— The older the eggs are the more likely it is that the embryo will have chromosome abnormalities, and again, it is normal;
— The influence of chromosomal errors on the morphology of the embryo (how the embryos look under the microscope) on 5-6 day of development is insignificant. It means that the embryo may look normal but have the abnormal set of chromosomes [Alfarawati et al., 2011].
What proportion of embryos at blastocyst stage is expected to have a normal set of chromosomes?
Diagram 1. Proportion of blastocysts with a normal set of chromosomes in women of different age groups according to S. Munne et al.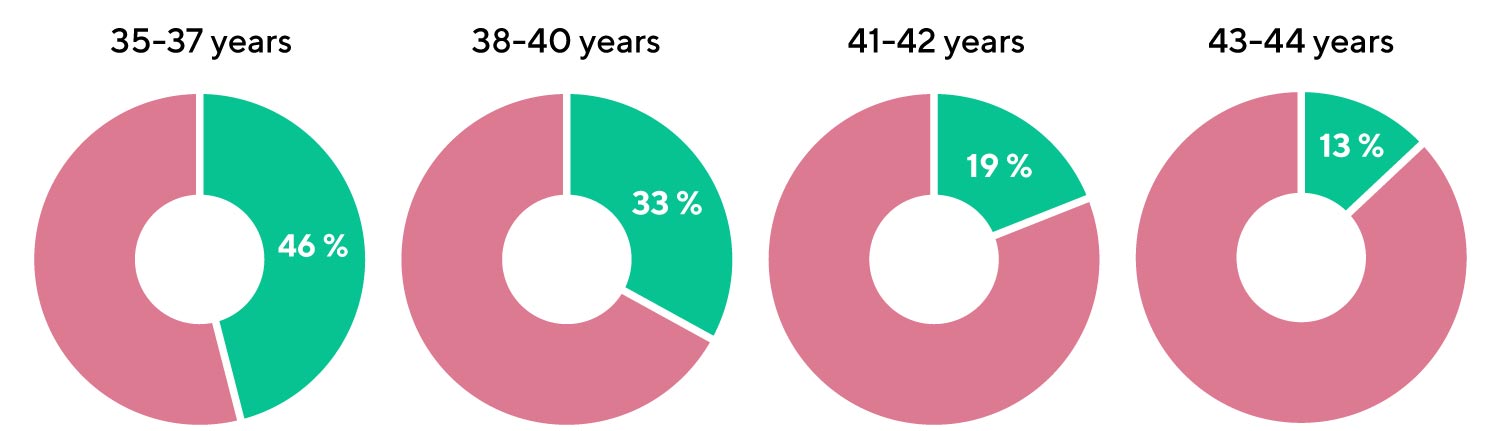
[S. Munne et al. Blastocysts needed to transfer at least one euploid embryo: data from 10,852 pre-implantation genetic screening (PGS) cycles. Fertility Sterility September 2015 Volume 104, Issue 3, Supplement, Pages e13–e14]
- Every second blastocyst is expected to have a normal set of chromosomes in the age group 35-37;
- Only one in 8 blastocysts is expected to have a normal set of chromosomes by the age of 43.
Diagram 2. Proportions of chromosomally normal blastocysts depending on the age of woman. O.L.G.A. Fertility data from 2019 to 2022.

In the data we collected in O.L.G.A. Fertility from 2019 to 2022 these proportions of chromosomally normal blastocysts depending on the age of woman are in line with an earlier study by S. Munne et al.
I.2. PGT-A — genetic testing of human embryos
Is there any way to identify which of the blastocysts with normal morphology (‘good-looking’) have a normal set of chromosomes and which have not?
What is PGT-A?
PGT-A — Preimplantation Genetic Testing for Aneuploidies (aneuploidyAneuploidy — an abnormal number of chromosomes. An abnormal number of chromosomes in cells of embryos, makes an embryo’s development stop. It is responsible for 70-80% of pregnancy losses in the first 12 weeks of pregnancy. — an abnormal set of chromosomes). This genetic testing of embryos locates the normal embryos, from those available. By excluding abnormal embryos from future use the live birth ratesLive birth rates — are calculated per embryo transfer. Live birth rates show percentage of embryo transfers which resulted in live birth are increased per embryo transfer. It happens because the testing identifies embryos (with the abnormal set of chromosomes) that would lead to implantation failures and pregnancy losses, and they are not transferred into the uterine cavity.
What is the purpose of PGT-A?
PGT-A does not make embryos better. PGT-A selects embryos with a normal set of chromosomes from those available to use them for the embryo transfer.
How is PGT-A carried out?
PGT-A tests the set of chromosomes in the DNA taken from embryonic placenta. Embryos with an abnormal chromosomal set will not lead to a healthy live birth, hence they are excluded from use. Embryos with a normal set of chromosomes are used for the embryo transfer.
What are the goals of PGT-A?
- To reduce miscarriage rates
- To increase live birth rates per one embryo transfer
- To shorten the time to a baby (reduce a number of embryo transfers before a baby is born)
What are the numbers?
Diagram 3. STAR1 Trial Compared with SART(National Summary report USA 2014 - 2017) Pregnancy outcomes

STAR — ongoing pregnancy rate per transfer, note that all these ongoing pregnancies resulted in a live birth
SART — live birth rate per one embryo transfer
The live birth rate per one embryo transfer of one embryo after PGT-A in our clinic is 51%. This means that in our clinic 51% of the transfers of a single embryo with a normal chromosome set lead to the birth of a child (Diagram 4).
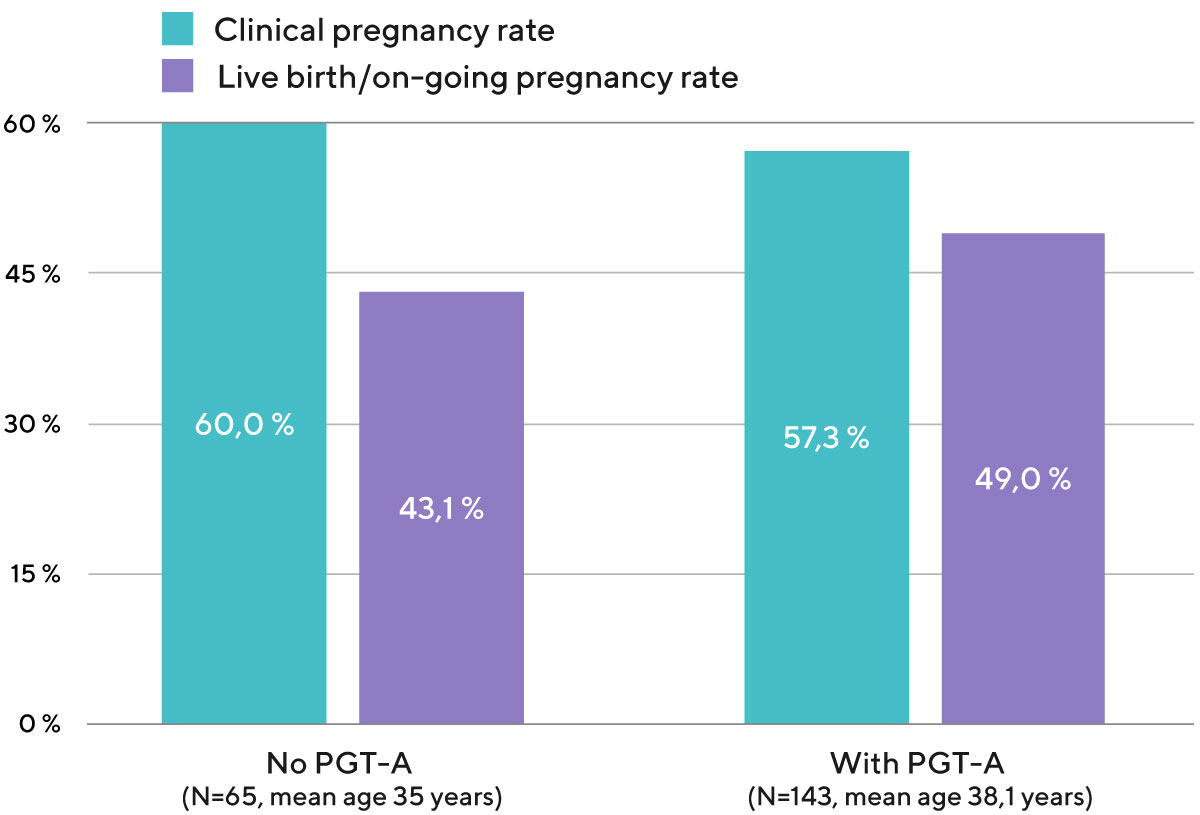
Diagram 4. Clinical pregnancy rate and live birth/on-going pregnancy rate per one embryo transfer with own eggs, depending on whether or not PGT-A was used to check the chromosomal status of the embryo (data from the embryo transfers performed from 2020 to 2022).
Who can benefit from using PGT-A to increase IVF success?
Women aged 35 and older have a significant increase in live birth rates per each embryo transferred after PGT-A, in comparison to control (no PGT-A). Also, in many cases (repeated pregnancy losses and implantation failures) PGT-A increases IVF success for women under 35 years old.
What is the process for biopsy and PGT-A?
Picture 2. Process of biopsy

- With a help of a pipette the embryologist takes a small piece of the embryonic external layer (future placenta);
- Immediately after the biopsy, the embryologist performs individual cryopreservation of each blastocyst;
- In the genetic laboratory, DNA is obtained from the cells of the embryonic placenta and is tested for 23 pairs of chromosomes, then the answer is given on which embryos have a normal chromosome count and which do not;
- Embryos with an abnormal number of chromosomes are excluded from future use;
- Embryos with a normal number of chromosomes can be used for the embryo transfer.
I.3. Q&A about PGT-A
My doctor in the Netherlands told me that US studies showed that sometimes chromosome abnormalities can fix themselves in early stages of development. Which means that genetic testing could potentially rule out embryos that could lead to pregnancies. Is it true?
It is true, embryos are dynamic structures and can change all the way. But the probability of PGT-A testing being wrong is pretty low, below 1-2%. But the benefit of testing is obvious in the older group of patients - you have much less chance that the pregnancy will not happen or that it will stop, leading to possible complications for the uterus and jeopardising your future chances for a baby, not to mention the psychological burden of that. Here I will be on the mother's side, even if it means not giving a chance for some potential babies. But this is a highly disputable topic nowadays, you can find arguments both pro and contra.
Dr. Anna Gusareva
In what genetic lab the PGT-A will be performed? Is it the Igenomix Laboratory in Spain? Will you perform the regular PGT-A or the new one from Igenomix called “Embrace”, where only the culture environment is analysed?
Usually PGT-A by NGS method is conducted in the Igenomix laboratory in Russia or Genetico lab also in Russia. We use these providers for a long period of time and are quite satisfied with the results. Blastocysts will be biopsied and frozen subsequently, the result we expect in 2 weeks. "Normal" PGT-A is the most reliable at the moment. Unfortunately Embrace results (testing of the spent culture media) are not so concordant with the real molecular karyotype of the embryo for now. We hope it will become more reliable in future but the current reality leaves us with only choice - if we need THE answer, trophectoderm biopsy + NGS is the best available approach. Our embryologists are very skilled in performing biopsy, we do it a lot, so you shouldn't worry about us harming the embryo in any way or diminishing its potential.
Dr. Anna Gusareva
I wonder if you can see in his sperm if there’s any defective genes coming from the sperm, for example ADHD or Asperger’s syndrome? I have read that the autism spectrum increases in the offspring the older the father is.
Unfortunately current level of science doesn’t give an opportunity to check spermatozoa for mutations before the fertilization. The checking could only be done on an embryo but only in such cases when we know that potential parents are known carriers of certain gene mutations. To check mutations in general is impossible for now.
Dr. Anna Gusareva
Have questions?
II. Maternal reasons for pregnancy loss and IVF failure
Maternal reasons for pregnancy loss and IVF failure can be of hormonal, inflammatory or immune nature. The reasons may be related to the state of the organs of the reproductive system or other organs and systems of the body. Often our patients have several reasons why an on-going pregnancy and live birth have not yet been achieved.
II.1. Missing the implantation window — the main reason of implantation failure in fresh IVF cycles
The process of embryo “sticking”, attaching in the uterus, is called implantation. Implantation starts with recognition of certain receptorsReceptor — is a protein structure on the surface of one cell, which recognizes a specific molecule or another receptor on the surface of another cell (like a key and lock). With the help of receptors cells, they can communicate with each other. An example of such dialogue: implantation dialogue between embryonic cells and endometrial cells during the implantation process on the surface of the endometrium by receptors on the surface of the hatched embryo.
The implantation window is the time frame in which these receptors are present in the endometrium, which is usually 5-7 days after the beginning of ProgesteroneProgesterone — the main pregnancy hormone. Progesterone production by the ovary begins at ovulation. Progesterone influences the endometrium and times its readiness for implantation — implantation window production by the ovulated follicle.
Normally, in a natural cycle, Progesterone production starts from the day of ovulation. Thus, usually the receptors on the endometrial cells are ready to meet with the embryo 5-7 days after ovulation. At the same time, the embryo, having carried out the process of hatching (coming out from the shell), is ready to meet the endometrial receptors 5-7 days after ovulation — on the 5-7th day of its development.
Nature has synchronized these two processes of embryonic and endometrial readiness for implantation very cleverly:
- The Embryo is ready to implant 5-7 days after ovulation (Picture 3).
- The Endometrium is ready for implantation 5-7 days after ovulation (Picture 4).
Picture 3. The Embryo is ready to implant 5-7 days after ovulation

Picture 4. The Endometrium is ready for implantation 5-7 days after ovulation
 Picture 4 illustrates how cleverly the nature synchronized process in the ovaries and uterus in a natural menstrual cycle.
Picture 4 illustrates how cleverly the nature synchronized process in the ovaries and uterus in a natural menstrual cycle.
FSH stimulates follicular growth from day 4 to 14
A follicle (a bubble with an egg inside) grows and produces Estrogen
Estrogen grows the lining in the uterus
Ovulation releases an egg and times the implantation window
Normally, Progesterone production starts immediately after ovulation
Progesterone is responsible for making the endometrium receptive to the embryo
Normally, the endometrium becomes receptive to the embryo 5-7 days after Progesterone production begins
The period when the endometrium is ready to recognize and receive the embryo is called the implantation window
The moment of the start of Progesterone production determines the implantation window.
However, ovarian stimulation may desynchronize these 2 processes: the readiness for implantation of the embryo and the endometrium. This happens due to supra-physiological levels of hormones in the body. In many stimulated cycles Progesterone production starts too early — already several days before egg retrieval (which is the equivalent of ovulation).
Should Progesterone productions start earlier than the day of embryo creation (egg retrieval or ovulation day), the receptors in the endometrium, which could have recognized the embryo, would be present earlier. When the embryo reaches the blastocyst hatchingHatching — a process of embryo leaving the eggshell. After hatching, a chicken would be able to simply walk away and take care of itself. A blastocyst takes care of itself in its own way too: its receptors look for receptors in the endometrium to attach to, in order to be able to continue living. stage, the receptors in the endometrium will no longer be there and this dialogue between the embryo and the endometrium will not be possible. Implantation will not take place!
Picture 5. Desynchronization of the embryo’s and endometrium’s readiness for implantation is one of the most frequent reasons for implantation failure in fresh IVF cycles
II.2. Restoring the dialogue between the embryo and the uterus by taking it one step at a time in your IVF process
This absence of dialogue between the embryo and the endometrium can be avoided by carrying out one task at a time.
The first step is to create competent eggs and viable embryos by using an individually tailored stimulation process. During this stimulation, we must focus only on the follicles, eggs and embryos. It is not possible to take care of the endometrium just yet.
When the blastocysts are ready, we stop time for them by freezing them gently. When we have stopped time for the embryos, we can then move our focus to the optimal preparation of the uterus ready for the embryo transfer.
For the embryo transfer we will choose a separate cycle in which we will focus on the endometrium: to transfer an embryo into the most optimal endometrium at the most optimal time. Usually, 1 menstrual cycle is necessary after the stimulated cycle for ovaries to stop producing residual amounts of Progesterone. Therefore, we recommend a one cycle break between the egg retrieval and embryo transfer.
Diagram 5. Cumulative live birth rate in all groups of patients who received embryo transfers within 1131 consecutive embryo transfers at O.L.G.A. Fertility (from 2019 to 2021)
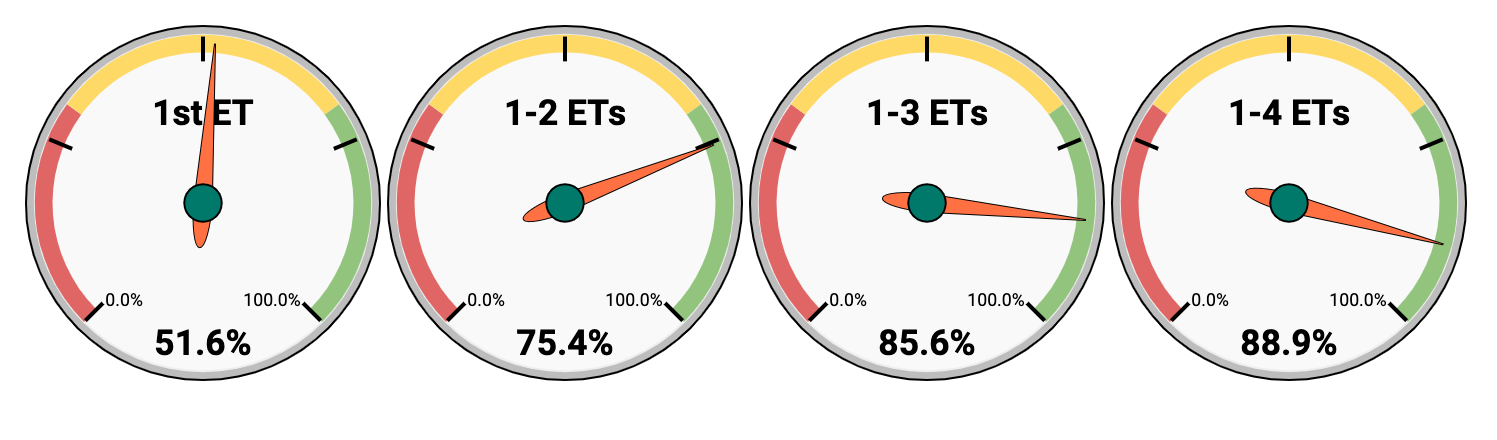
In O.L.G.A. Fertility you have:
- 51.6% chance of live birth after 1 ET
- 75.4% chance of live birth after 2 ETs
- 85.6% chance of live birth after 3 ETs
- 88.9% chance of live birth after 4 ETs
As a result, if we persevere and consistently move towards the goal, the chance of having a baby after 4 embryo transfers in O.L.G.A. Fertility is 88.9%.
You can read more about our IVF Process in the following chapter →
Have questions?
II.3. Q&A
- Are autoimmune diseases in the mother can be the reason for pregnancy loss?
- In case of egg donation, is there a higher risk of pregnancy loss due to immunologic reasons as the egg is not own but “foreign material”?
- I wonder why don’t the embryo / blastocyst attach to the uterus?
- I have gotten one child through stimulation via egg follicles and then I got naturally pregnant. I got a planned cesarean. If I want to have a baby again by embryo adoption due to my age of 46, do you think I have scar tissue after the cesarean? What is the chance you need to do a hysteroscopy of my uterus? Is it something I should plan to do before the first embryo adoption attempt?
- Hello, can this ERA Test be done in the Test cycle locally at my gyn doctor or in a fertility clinic in Germany? It seems to be a key to the right timing!
- If a hysteroscopy is recommended before any treatment, how much does it cost and/or is it part of a treatment plan?
- I am wondering if there are any nutritional suggestions for us that have multiple failed implantations and miscarriages on the ones that do implant.
- Do you always wait another cycle after extracting the egg before implanting it? Some fertility clinics want to do the implant on the same cycle. Why does this differ between different clinics and why do you choose not to do it in the same cycle?
- Research in how Covid-19 can affect an embryo. I had a miscarriage in early pregnancy and most likely I was infected by the virus.
Are autoimmune diseases in the mother can be the reason for pregnancy loss?
Yes, maternal autoimmune diseases do increase the likelihood of losing a pregnancy. But this does not mean that nothing can be done. We recommend planning a pregnancy when an autoimmune disease is stable, not in the acute stage. We also recommend additional medicine to minimize the aggressive effect of autoantibodies on trophoblast (future placenta). This supporting therapy has to be selected individually for each patient.
Dr. Elena Lapina
Back to Index
In case of egg donation, is there a higher risk of pregnancy loss due to immunologic reasons as the egg is not own but “foreign material”?
Embryos are always “foreign” for the mother’s body, and it doesn’t matter if we use the mother’s eggs or not. The embryo is a completely new organism with its own unique set of characteristics. The incidence of spontaneous pregnancy loss in patients under 35 years of age (when using their own eggs) and the incidence of spontaneous pregnancy loss in older women using donor eggs (with donor’s age up to 35 years) is the same. With own eggs in women under 35, the miscarriage rate is — 10 %, with donor oocytes — 9.2% (our clinic’s own data).
Dr. Elena Lapina
Back to Index
I wonder why don’t the embryo / blastocyst attach to the uterus?
There can be several reasons for implantation failure. Firstly, the embryo may have a wrong chromosome set, which makes the embryo’s development stop (for example right after getting into the uterus). Secondly, in the uterine cavity, there is often a specific issue that reduces endometrial receptivity (such as adenomyosis, inflammation, etc.). Often, these causes are not visible with just ultrasound. Thirdly, the dialogue between the embryo and the endometrium may be impaired. The embryo might be wonderful and the endometrium might be overall ready, but not at the specific time when the embryo transfer took place. Instead, it might be a little earlier or a little later. In each individual case, these causes may occur as just one, or as a combination of them. Studying the patient’s history of previous conception attempts, we can suspect one or another.
Dr. Elena Lapina
Back to Index
I have gotten one child through stimulation via egg follicles and then I got naturally pregnant. I got a planned cesarean. If I want to have a baby again by embryo adoption due to my age of 46, do you think I have scar tissue after the cesarean? What is the chance you need to do a hysteroscopy of my uterus? Is it something I should plan to do before the first embryo adoption attempt?
Scarring does not always occur after cesarean section. A big problem for subsequent implantation is a cesarean scar defect, or niche. Menstrual blood accumulates in this niche and stays there for quite some time, sometimes even until ovulation. This remaining blood causes inflammatory changes in the endometrium and changes endometrial receptivity. Fortunately, it is fairly easy to notice on an ultrasound scan that a niche has formed after the cesarean section.
You can confirm its presence with hysteroscopy as well, and, quite often, with hysteroscopy, you can also improve the outflow of menstrual blood from a niche by removing scarring around. In just 2 months after such hysteroscopy, it is possible to schedule an embryo transfer. I want to emphasize once again that the history of a C-section does not always affect endometrial receptivity. After analyzing the results of ultrasound examination and finding out the specifics of your menstrual cycle, we can give you an individual recommendation on the necessity for hysteroscopy.
Dr. Elena Lapina
Back to Index
Hello, can this ERA Test be done in the Test cycle locally at my gyn doctor or in a fertility clinic in Germany? It seems to be a key to the right timing!
We are ready to take into account the results of the ERA test performed in other clinics. If you are planning to undergo fertility treatment in our clinic, please check with your doctor at our clinic what you need to do to prepare for the ERA test. This is very important because the ERA test has to be done under the same conditions as the future embryo transfer.
Dr. Elena Lapina
Back to Index
If a hysteroscopy is recommended before any treatment, how much does it cost and/or is it part of a treatment plan?
We recommend hysteroscopy individually if we suspect an abnormality after doing an ultrasound or when analyzing your medical history; we may suspect an abnormality based on your history analysis even despite good ultrasound pictures. Depending on the treatment packages hysteroscopy may be included or cost extra.
Dr. Elena Lapina
Back to Index
I am wondering if there are any nutritional suggestions for us that have multiple failed implantations and miscarriages on the ones that do implant.
We recommend taking folic acid as it helps control homocysteine levels. With high homocysteine levels, pregnancy loss is more likely due to the formation of tiny blood clots in small arteries (micro-thrombosis). Besides, please look into taking Omega 3 and Vitamin D. They are immune-modulators that help in normalizing the implantation process.
Dr. Elena Lapina
Back to Index
Do you always wait another cycle after extracting the egg before implanting it? Some fertility clinics want to do the implant on the same cycle. Why does this differ between different clinics and why do you choose not to do it in the same cycle?
When stimulation is prolonged for the sake of a competent egg, endometrium may become too old and lose its interest in implantation. You cannot have everything at once. Sometimes you need to split a complex project in several steps. We prefer the step-by-step approach - first optimized stimulation protocol with egg retrieval and creation of embryos. Second — correct assessment and preparation of your endometrium to achieve best implantation potential. The third – transfer of a competent embryo into an optimally prepared endometrium.
Dr. Anna Gusareva
Back to Index
Research in how Covid-19 can affect an embryo. I had a miscarriage in early pregnancy and most likely I was infected by the virus.
I am so very sorry that such a sad thing happened. But there is not enough data for now to give a correct answer to your question. We simply don't possess the information about the influence of COVID-19 on early stages of pregnancy. It seems that those women who were in their third trimester of pregnancy and become infected with the virus don't pass the virus to their babies, they were born healthy. And pregnancy doesn't affect the chances of a woman to be cured. But the medicines which are currently used for the treatment of COVID-19 are not recommended during pregnancy, so treatment itself can have a negative impact on the baby.
Dr. Anna Gusareva
Back to Index